
Apparently, their active biomolecules most likely comprise amino, hydroxyl, and carboxyl biofunctional groups which would function a dual role as a reductant of metal salts and as protective agents to form a stabilizing layer on the biosynthesized nanoparticles. Owing to presence highly active bio-organic molecules in plants, eco-friendly plant-based methodologies such as roots, flowers, seeds, leaves, bark, and fruits, have emerged as extremely promising biological factories for the formation of the diverse range of inorganic Hence, recent researches have been attempted to employ nature-based sources such as plants extracts, fungi, bacteria, and marine organisms, as sustainable alternative methods toward green nanoparticles fabrication. Whereas biological procedures are non-toxic, high atom economy, straightforward methodology and most importantly they are environment-friendly. solvent, the reducing agent, acid and base reagents) which potentially expose the environment to serious health hazards so that restricts their advantages. Despite their popularity, they are high cost and often contain toxic materials (e.g.

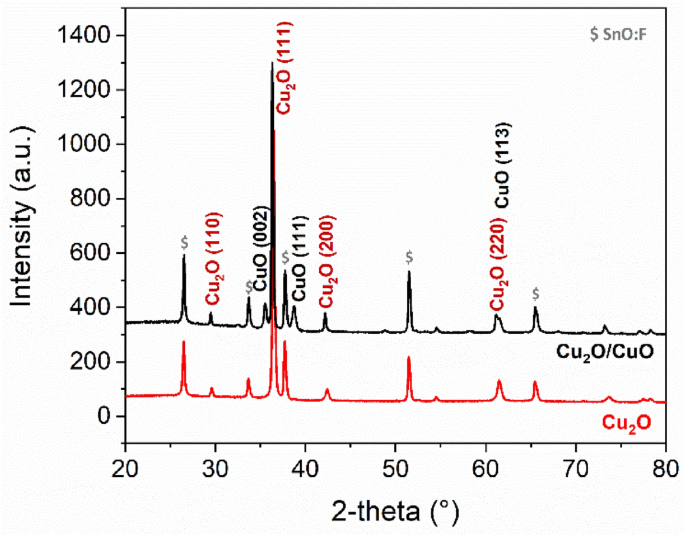
Presently, a considerable number of physical and chemical procedures were presented to fabricate the various types of nanoscale particles with different shape and size. Nanoparticles owing to high surface area and a tiny particle size show inimitable material characteristics in comparison with bulk materials, which make them high in demand and widely applied area for miscellaneous applications in recent years. Bio-assisted copper oxide nanoparticles demonstrated significant catalytic efficiency and reusability toward 4-nitrophenol removal by an average of 97.6% from aqueous solutions after successive 5 days’ exposure to UV irradiation. X-ray photo-electron spectroscopy analyses revealed only copper and oxygen elements in the sample, confirming the purity of copper oxide nanoparticles. Transmission electron microscopy results showed the spherical shape of nano-particle with an average size of 22 ± 1.5 nm. The stability of copper oxide nanoparticles was confirmed after 3 months’ storage of product with no sedimentation or suspension. X-ray diffraction pattern results demonstrated the monoclinic structure of highly pure biosynthesized copper oxide nanoparticles with a crystallite size of 20.76 nm. Fourier-transform infrared spectroscopy analysis determined Cu–O bonds in nanosample, indicating the active role of functional groups in the wheat seed extract in bio-reduction of Cu cations. The ultraviolet-visible absorption peak at 300 nm was confirmed the formation of copper oxide nanoparticles. Under optimal reaction conditions, the wheat seed extract-derived electron-rich biomolecules were functioned as a reducing and capping/ stabilizing agent. The synergistic effect between copper and cerium was the predominant contributor to the improved catalytic activities of CuO-CeO 2 catalysts, instead of structural properties.A facile novel green methodology is presented for the synthesis of highly stable and well-dispersed copper oxide nanoparticles using aqueous wheat seed extract.

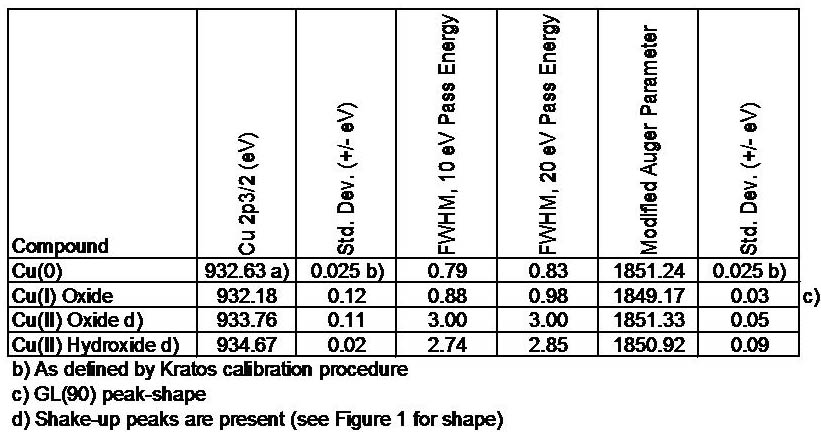
(2) In contrast, the catalysts from Ce(III) precursor without excellent texture displayed high reducibility and activities for CO oxidation due to the high content of Ce 3+, following the redox equilibrium of Cu 2+ + Ce 3+ ↔ Cu + + Ce 4+ shifting to right to form more stable Cu + species, which was the origin of synergistic effect. The obtained results suggested that the precursors exerted a great influence on the properties of CuO-CeO 2 catalysts: (1) compared with the catalysts from Ce(III) precursor, the derived Ce(IV) precursor catalysts showed smaller grain size, higher BET surface area, narrower pore size distribution, whereas their reducibility and activities were not enhanced. The catalysts were characterized by TG–DTA, XRD, LRS, N 2 adsorption–desorption, HRTEM, XPS, H 2-TPR and in situ FT-IR. This work investigated the effects of cerium precursors on the structure, surface state, reducibility and CO oxidation activity of mesoporous CuO-CeO 2 catalysts.


 0 kommentar(er)
0 kommentar(er)
